Stability Studies with Cryo-TEM
Understand the impact of storage conditions (buffer, pH, temperature, time) on size distribution, shape & morphology, lamellarity, and other CQAs of nanoparticle formulations such as:
- Vaccines
- Lipid Nanoparticles & liposomes
- Viral & VLP formulations
- Gene & drug delivery systems
Robust Formulation Stability Assessments
Comprehensive analysis of the effects of storage conditions (buffer, pH, temperature, time) on formulation morphology is crucial for maintaining formulation stability.
Stability studies with cryo-TEM imaging can be done at any phase, and data generated from validated methods can be used for regulatory filings.

Samples we can Perform Stability Testing on

- Adeno-Associated Virus (AAV)
- Adeno-Associated Virus (AAV)

- Adenovirus
- Adenovirus

- Bacteriophage
- Bacteriophage

- Extracellular Vesicles & Exosomes
- Extracellular Vesicles & Exosomes

- Gold Nanoparticles
- Gold Nanoparticles

- Human Papillomavirus (HPV)
- Human Papillomavirus (HPV)

- Iron Nanoparticles
- Iron Nanoparticles
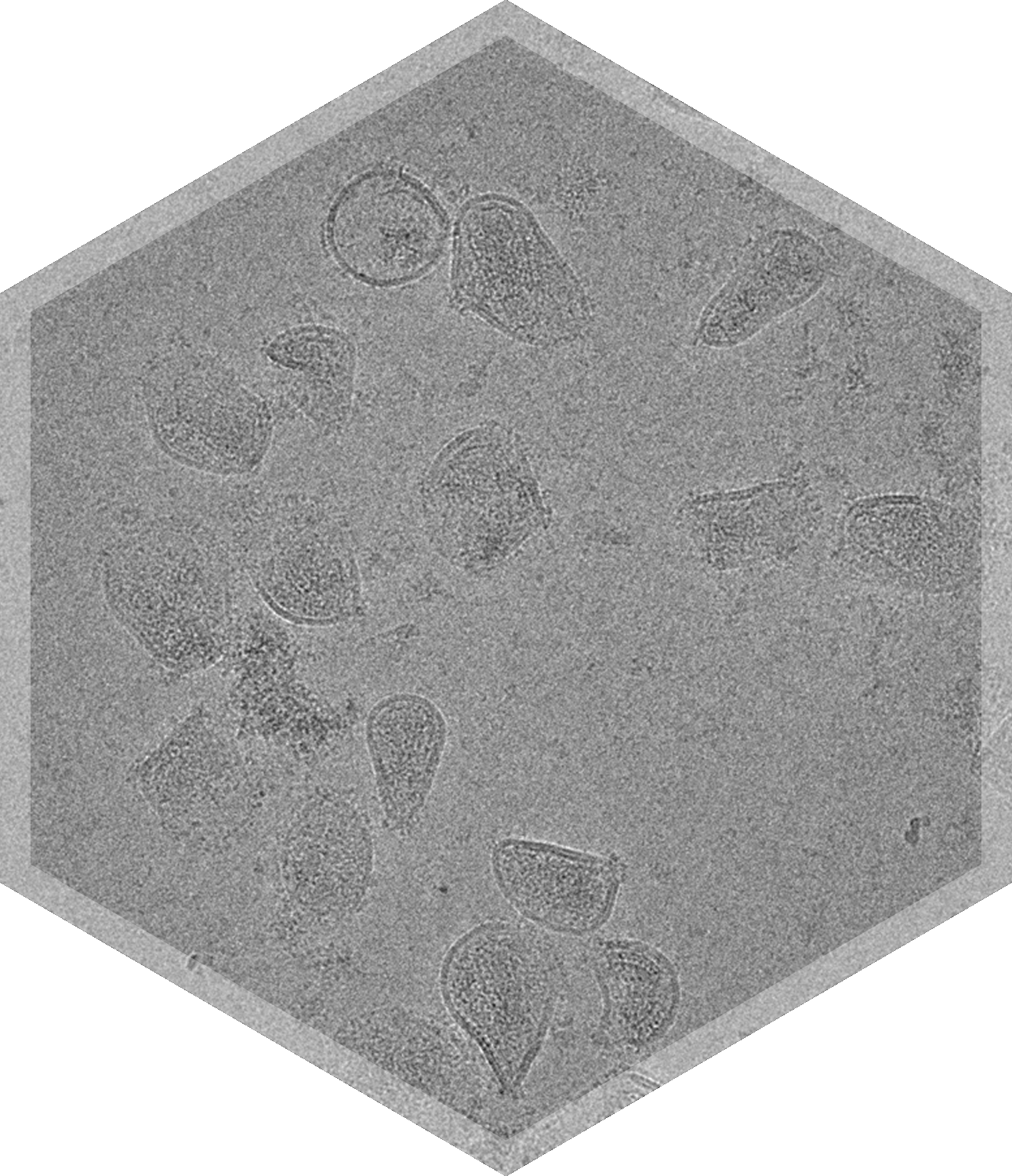
- Lentivirus
- Lentivirus

- Lipid Nanoparticles (LNPs), with mRNA, RNA, DNA payloads
- Lipid Nanoparticles (LNPs), with mRNA, RNA, DNA payloads

- Liposomes
- Liposomes

- Micelles
- Micelles

- Other Nanoparticle Samples
- Other Nanoparticle Samples

- Polymeric Nanoparticles
- Polymeric Nanoparticles

- Protein Based Nanoparticles
- Protein Based Nanoparticles

- Two Component Systems
- Two Component Systems

- Vesicular Stomatitis Virus
- Vesicular Stomatitis Virus

NIS helps visualize critical quality attributes directly
The thermal stability of liposomes, lipid nanoparticles, and other nanoparticles often reflects their in vivo stability and drug release characteristics. These properties can be adversely influenced and affected by the added components, including the drug itself, making it important to understand the structure and dynamics of the system as a whole. Stability is a critical quality attribute that must be controlled during drug development and manufacturing, through to final product use.
Transmission Electron Microscopy (TEM), and in particular, cryo-TEM allows for the direct visualization of particles and a comprehensive characterization of morphology to monitor and ensure product stability. The direct analysis and characterization of nanoparticles with cryo-TEM allows for monitoring the effect of changes to the size distribution, shape & morphology, and lamellarity from external variables such as product contamination and storage on particle stability. Cryo-TEM can also help evaluate the influence of storage conditions, including pH, temperature, and time, on the morphology on samples such as lipid nanoparticles.






.png)
.png)
.png)
.png)

